Kenyans could be among the first in Africa to benefit from the revolutionary injection once it’s available and accessible.
A major partnership this week between the Gates Foundation and Indian drug company, Hetero Labs, will boost Kenya’s fight against HIV, as the country will be among the first in Africa to receive a low-cost, generic version of Lenacapavir, the twice-yearly HIV prevention jab.
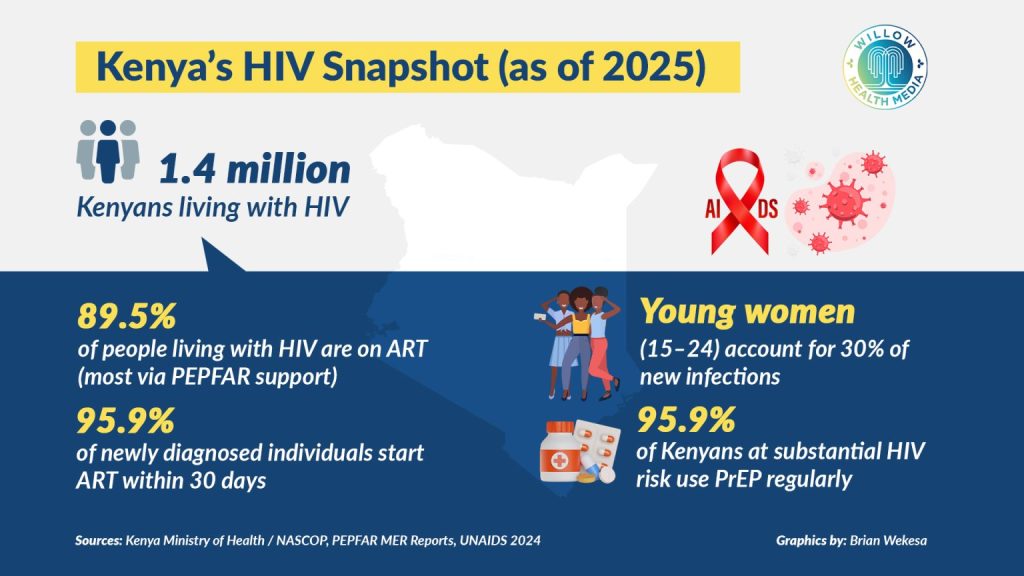
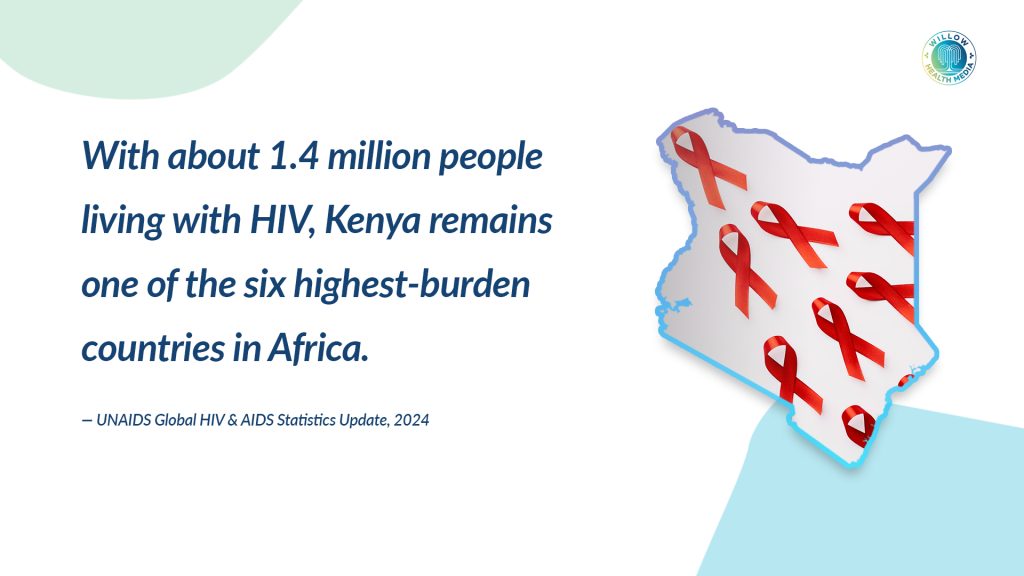
Kenya has about 1.4 million people living with HIV and 20,000 new infections annually, according to UNAIDS. While daily prevention pills are more available now, many people, especially in rural communities, struggle to use them consistently due to stigma and limited access.

Lenacapavir, which was approved by the US Food and Drug Administration (FDA) in June and the European Commission in August, is the world’s first HIV prevention drug requiring only two shots annually. For Kenyans living far from clinics and who find it hard to take daily oral pre-exposure prophylaxis (PrEP) pills, this could be a lifesaving solution.
Dr Vamsi Krishna, MD of Hetero Labs, said the collaboration reflected their “commitment to ensuring access to innovative HIV medicines for patients in India and other low- and middle-income countries.”

The drug’s cost is pegged at around $40 (Ksh5,200) per patient annually after a short pre-treatment oral regimen, which is affordable for Kenya’s public health system.
The Gates Foundation is partnering with global health organisations, including Dr Reddy’s Laboratories, Unitaid and Clinton Health Access Initiative (CHAI) to create a competitive market for the generic Lenacapavir in readiness for large-scale production by 2027, pending approvals.
Drug could be transformative for Kenya’s young people, who account for more than half of new HIV infections
Kenya is well-positioned to be an early beneficiary as Lenacapavir maker, Gilead Sciences, has allowed six generic manufacturers to produce it for 120 low-income countries, including Kenya, which has a strong existing network for HIV care from partners like the Global Fund and the US President’s Emergency Plan for AIDS Relief (PEPFAR).
“The deals announced today on generics are a major step forward in ending the HIV epidemic,” said Kate Hampton, CEO of the Children’s Investment Fund Foundation (CIFF). “They build on full value-chain investments to foster a competitive market so that access to Lenacapavir is affordable and reliable for all those who need it.”

While Kenya has made substantial progress in reducing HIV prevalence over the past two decades, challenges remain. The Ministry of Health has scaled up PrEP use in key populations like young women and adolescent girls, who face disproportionate risks of infection. However, fewer than one in five people who could benefit from PrEP are currently accessing it.
Studies show that introducing Lenacapavir to just four per cent of the population in high-risk countries could cut new infections by 20 per cent. For Kenya, where young people account for more than half of new infections, the impact could be transformative.

The twice-yearly shot solves the problem of daily pills, as Kenyan patients would only need two annual clinic visits
“Lenacapavir can help us end the HIV epidemic if they are made accessible to people who can benefit from it the most,” said Trevor Mundel, President of global health at the Gates Foundation. “We are committed to ensuring that those at highest risk, who can least afford it, aren’t left behind.”
This announcement follows a $912 million pledge to the Global Fund’s 2026–2028 budget from the Gates Foundation to support efforts to save 23 million lives from HIV, malaria, and tuberculosis between 2027 and 2029.
For Kenya, low-cost generic Lenacapavir could stretch limited resources further and increase programme reach.
The Hetero agreement also covers supply of the drug’s active component ingredient (API), opening doors for local and regional manufacturers, including African manufacturers, even in Kenya, to produce it in the future.
For Kenyans in rural and underserved areas, a twice-yearly shot solves the main problem of daily pills, as patients would only need to make two clinic visits a year, which is much easier to manage with the current healthcare system.
Once approved and available, the Kenyan government will add Lenacapavir into Kenya’s HIV prevention toolkit, marking a turning point in accelerating the decline of new infections, moving the country closer to ending the epidemic.
For millions of Kenyans at risk, this isn’t just a scientific breakthrough; it is a chance at a healthier, HIV-free future.