‘We’re acting with determination to rapidly halt the spread of the virus and protect communities’- Dr Mohamed Janabi, WHO Regional Director for Africa
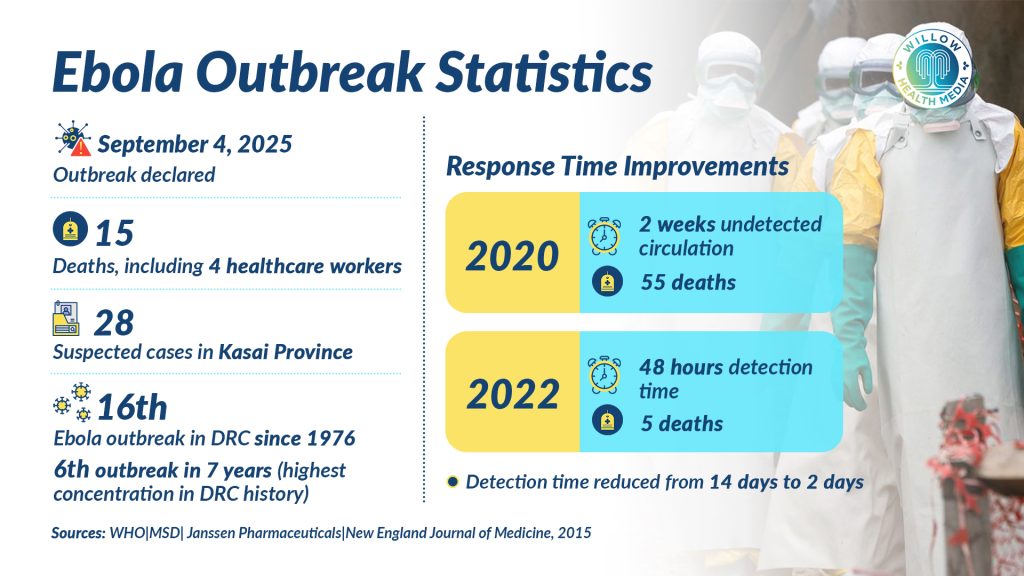
The Democratic Republic of the Congo (DRC) has declared its 16th Ebola outbreak since 1976, with health authorities confirming 28 suspected cases and 15 deaths in the remote Kasai Province. Four healthcare workers have already died in the outbreak announced on September 4, 2025.
The outbreak has affected the Bulape and Mweka health zones in Kasai Province, with samples tested on September 3 confirming the cause as the deadly Ebola Zaire strain – the same strain responsible for the 2014-2016 West Africa epidemic that killed over 11,000 people.

“We’re acting with determination to rapidly halt the spread of the virus and protect communities,” said Dr Mohamed Janabi, WHO Regional Director for Africa. “Banking on the country’s long-standing expertise in controlling viral disease outbreaks, we’re working closely with the health authorities to quickly scale up key response measures to end the outbreak as soon as possible.”
This emergency marks the sixth outbreak in seven years – the highest concentration in the nation’s history. The DRC’s relationship with Ebola spans nearly five decades, with an alarming increase in outbreak frequency since 2018. Previous outbreaks in Kasai Province occurred in 2007 and 2008, but neither reached today’s scale of concern.
The most significant outbreak occurred between 2018 and 2020 in North Kivu and Ituri provinces, becoming the second-largest Ebola epidemic in history with 3,481 total cases and 2,299 deaths. Its duration of over two years was complicated by active conflict zones.
DRC has 2,000 doses of ERVEBO vaccine against the Zaire strain of Ebola
Despite recurring outbreaks, the DRC has demonstrated remarkable improvement in response capabilities. Detection times have dramatically decreased from two weeks of undetected circulation in 2020 (resulting in 55 deaths) to detection within 48 hours in 2022 (resulting in only five deaths). This improvement reflects investments in community-based surveillance, laboratory networks, and real-time reporting systems.
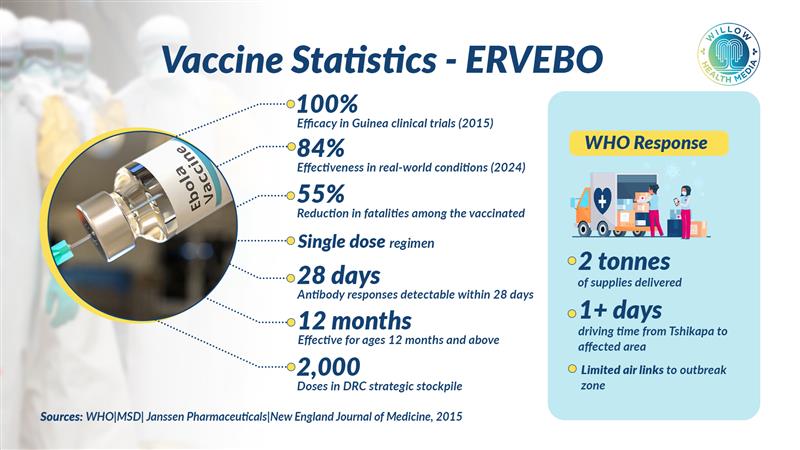
The country now maintains a strategic stockpile of 2,000 doses of the ERVEBO vaccine, specifically effective against the Zaire strain identified in the current outbreak. The vaccine, which demonstrated 100 per cent effectiveness in clinical trials when administered more than 10 days before exposure, will be rapidly deployed from Kinshasa using proven ring vaccination strategies to vaccinate close contacts and frontline healthcare workers.
The WHO is delivering two tonnes of supplies, including personal protective equipment, mobile laboratory equipment, and medical supplies. However, the area is difficult to reach, taking at least one day of driving from Tshikapa (the provincial capital of Kasai), with few air links. The WHO has deployed a comprehensive response team including epidemiologists, infection prevention specialists, laboratory experts, and risk communication professionals.
Patients have presented with symptoms including fever, vomiting, diarrhoea, and haemorrhage. Samples were tested at the country’s National Institute of Biomedical Research in Kinshasa.
Deployment of Ebola vaccines is one of the most significant advances in infectious disease control of the 21st century
While the outbreak remains localised, health security experts are monitoring factors that could influence its trajectory. Kasai Province’s location necessitates surveillance of neighbouring regions and trade routes that could facilitate the spread of the virus. WHO predicts case numbers are expected to increase through expanded surveillance efforts, making the coming weeks crucial in determining whether rapid containment can be achieved.
The development and deployment of Ebola vaccines represents one of the most significant advances in infectious disease control of the 21st century. ERVEBO (rVSVΔG-ZEBOV-GP) by Merck Sharp & Dohme, the first licensed Ebola vaccine, achieved regulatory approval through accelerated pathways based on compelling field trial data. It received European Medicines Agency authorisation in November 2019 and US FDA approval in December 2019.
ERVEBO is a live, attenuated recombinant vesicular stomatitis virus vector expressing the Zaire Ebola virus glycoprotein, indicated for individuals aged 12 months and above. It requires a single dose, with antibody responses detectable within 28 days. Clinical data from the 2015 ring vaccination trial in Guinea demonstrated 100 per cent efficacy when administered more than 10 days before exposure, while a 2024 study showed 84 per cent effectiveness in real-world conditions. DRC data show a 55 per cent reduction in case fatality rates among vaccinated individuals.
The vaccine’s approval extends to multiple African countries at endemic risk, including the DRC, Ghana, Guinea, Rwanda, Uganda, Burundi, the Central African Republic, the Republic of Congo, Sierra Leone, and Zambia, enabling rapid deployment during outbreaks.
The Zabdeno/Mvabea regimen by Janssen Pharmaceuticals uses a heterologous prime-boost strategy, with an adenovirus 26 vector (Zabdeno) followed by a modified vaccinia virus Ankara vector (Mvabea), administered 56 days apart. Designed primarily for preventive vaccination in lower-risk populations, it induces strong humoral immunity with responses persisting for at least two years and potential cross-protection against multiple Ebola virus species.
Ebola vaccine challenges include hesitancy, cold chain storage, coordination in conflict areas
Global vaccine stockpiles, managed by the International Coordinating Group, facilitate rapid deployment. Since 2021, over 145,000 doses have been distributed, with most used for preventive vaccination.
Significant limitations remain, including knowledge gaps regarding long-term immunity, booster requirements, and protection in vulnerable populations such as infants under 12 months, pregnant women, immunocompromised individuals, and the elderly. Deployment challenges include vaccine hesitancy, cold chain maintenance, and coordination in resource-limited or conflict-affected areas.
The success of ERVEBO and Zabdeno/Mvabea in achieving high efficacy, reducing case fatality rates, and enabling rapid outbreak containment demonstrates the power of sustained investment in vaccine research, international cooperation, and evidence-based public health interventions.
The current outbreak in Kasai Province will test these improved response capabilities, emphasising the ongoing need for broader-spectrum vaccines, optimised deployment strategies, and sustainable health system strengthening to ensure global health security. The world watches as the DRC once again confronts an ancient enemy, armed with better tools and deeper experience than ever before, but facing the persistent challenges of geography, resources, and the unpredictable nature of viral disease transmission.
This story was first published by Willow Health Media on September 6, 2025.